Sorry, we did not find an exact match.
Suggestions
We are constantly improving our dictionaries. Still, it is possible that some words are not available. You can ask other members in forums, or send us email. We will try and help.
Atomic Number 42
SHABDKOSH Apps
42 is the mass number and 20 is the atomic number. Explanation: The mass number of an element is written at the upper left side of the symbol of the element, and the atomic number is written at the lower left side of the symbol. 42 is the atomic number of molybdenum. 42 is the atomic mass of one of the naturally occurring stable isotopes of calcium. The angle rounded to whole degrees for which a rainbow appears (the critical angle).
Recent Search History
See and manage historyEnglish to Sanskrit Dictionary: atomic number 42
Meaning and definitions of atomic number 42, translation of atomic number 42 in Sanskrit language with similar and opposite words. Spoken pronunciation of atomic number 42 in English and in Sanskrit.
Tags for the entry 'atomic number 42'
What atomic number 42 means in Sanskrit, atomic number 42 meaning in Sanskrit, atomic number 42 definition, explanation, pronunciations and examples of atomic number 42 in Sanskrit.
Also see: atomic number 42 in Hindi
Our Apps are nice too!

Dictionary. Translation. Vocabulary.
Games. Quotes. Forums. Lists. And more...
Try our vocabulary lists and quizzes.
Snacks
Fruits
Sports
We provide a facility to save words in lists.
Atomic Number 42 In Periodic Table
Custom Word Lists
You can create your own lists to words based on topics.
Login/Register

To manage lists, a member account is necessary.
Atomic Number And Mass Chart
Member Account.
Support
Keep in Touch
Get our Apps
- © 2021 Shabdkosh.com, All rights reserved.
- Fast, Free and Offline
- Over 100,000 words
- Audio Pronunciation
- Word Games
- Word & Quote of the Day
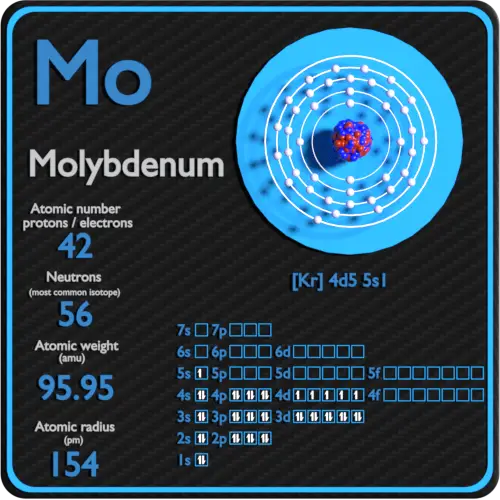
Atomic Number of Molybdenum is 42.
Chemical symbol for Molybdenum is Mo. Number of protons in Molybdenum is 42. Atomic weight of Molybdenum is 95.95 u or g/mol. Melting point of Molybdenum is 2617 °C and its the boiling point is 5560 °C.
Molybdenum Element
» Boiling Point» Melting Point» Abundant» State at STP» Discovery YearAbout Molybdenum
Molybdenum is a shiny grey color metal, with the name deriving from the Greek word meaning leadlike. Despite being toxic, this element plays an important role for all living organisms and is present in our bodies in micro doses. It is processed from natural minerals (mainly molybdenite) which are found mainly in South and North America, or in China. Molybdenum is an element with high melting point, so most of it comes in the form of grey powder. In industry, this element is used as a part of alloys, to produce such parts as saw blades, drills, heating parts, and many others. Molybdenum can also be used as a catalyst and a lubricant additive.
Uses of Molybdenum
Molybdenum which is a gray metallic element with the symbol Mo is mainly used as an alloying agent in the production of ferrous and steel alloys. The major use of molybdenum is in metallurgy, the rest is used in chemical applications. Because of its strength and corrosion resistance, molybdenum is especially used in jet engines, heating elements, drills, missiles, saw blades, and combustion liners. It also is employed in the petroleum industry too. Besides, molybdenum powder can be employed as a fertilizer.
Compounds with Molybdenum
- Na2MoO4: Sodium molybdate
- MoS2: Molybdenum disulfide
- (NH4)2MoO4: Ammonium molybdate
- MoO3: Molybdenum trioxide
- PbMoO4: Wulfenite
- MoSi2: Molybdenum disilicide
- MoF5: Molybdenum (V) fluoride
- MoF6: Molybdenum hexafluoride
- Mo(CO)6: Molybdenum hexacarbonyl
- Cl4Mo: Molybdenum tetrachloride
Properties of Molybdenum Element
| Atomic Number (Z) | 42 |
|---|---|
| Atomic Symbol | Mo |
| Group | 6 |
| Period | 5 |
| Atomic Weight | 95.95 u |
| Density | 10.22 g/cm3 |
| Melting Point (K) | 2896 K |
| Melting Point (℃) | 2617 °C |
| Boiling Point (K) | 4912 K |
| Boiling Point (℃) | 5560 °C |
| Heat Capacity | 0.251 J/g · K |
| Abundance | 1.2 mg/kg |
| State at STP | Solid |
| Occurrence | Primordial |
| Description | Transition metal |
| Electronegativity (Pauling) χ | 2.16 |
| Ionization Energy (eV) | 7.09243 |
| Atomic Radius | 145pm |
| Covalent Radius | 145pm |
| Valence Electrons | 1 |
| Year of Discovery | 1778 |
| Discoverer | Scheele |

What is the Boiling Point of Molybdenum?
Molybdenum boiling point is 5560 °C. Boiling point of Molybdenum in Kelvin is 4912 K.
What is the Melting Point of Molybdenum?
Molybdenum melting point is 2617 °C. Melting point of Molybdenum in Kelvin is 2896 K.
How Abundant is Molybdenum?
Abundant value of Molybdenum is 1.2 mg/kg.
What is the State of Molybdenum at Standard Temperature and Pressure (STP)?
State of Molybdenum is Solid at standard temperature and pressure at 0℃ and one atmosphere pressure.
When was Molybdenum Discovered?
Molybdenum was discovered in 1778.
