Perrin named the number Avogadro's number, not Avogadro's constant. This name had continued till 1971. In 1971, the International System of Unit (SI) introduced a new quantity called Avogadro's constant. The Avogadro constant has the same numerical value as the Avogadro number, but they differ in the unit which will be explained later in this. Here is the answer for the question – Avogadro’s Number or NA. You’ll find the correct answer below Avogadro’s Number or NA The Correct Answer is 6.02×10^23Ex: 3 mol carbon x 6.02 x 10^23/ 1 mol C atoms Reason Explained 6.02×10^23Ex: 3 mol carbon x 6.02 x 10^23/ 1 mol C atoms is correct for. Avogadro’s number is 6.022 x 10 23. It is the value given for the Avogadro’s Constant. The same thing represented by the Avogadro’s number was first calculated by a German scientist Johann Loschmidt but in different units. Here is the answer for the question – Avogadro’s Number or NA. You’ll find the correct answer below Avogadro’s Number or NA The Correct Answer is 6.02×10^23Ex: 3 mol carbon x 6.02 x 10^23/ 1 mol C atoms Reason Explained 6.02×10^23Ex: 3 mol carbon x 6.02 x 10^23/ 1 mol C atoms is correct for. How many moles of Na contain 1.45x10 21 atoms of Na? (to find moles, divide atoms by Avogadro's number) answer choices.
| Value of NA[1] in various units |
|---|
| 6.02214179(30)×1023 mol−1 |
| 2.73159757(14)×1026 lb-mol−1 |
| 1.707248479(85)×1025 oz-mol−1 |
The Avogadro constant (symbols: L, NA) is the number of particles (usually atoms or molecules) in one mole of a given substance.[2] Its value is equal to 6.02214129(27)×1023 mol−1.[3] The constant was named after the ItalianscientistAmedeo Avogadro.
The measurement of Avogadro's constant was refined in 2011 to 6.02214078×1023 ± 0.00000018×1023.[4]
An old term closely related to the Avogadro constant is Avogadro's number. Avogadro's number is the number of atoms in 12 grams of the carbonisotopecarbon-12. Avogadro's number is a dimensionless quantity and has the numerical value of the Avogadro constant given in base units.

Related pages[change | change source]
References[change | change source]
- ↑Mohr, Peter J. (2008). 'CODATA Recommended Values of the Fundamental Physical Constants: 2006'(PDF). Rev. Mod. Phys.80: 633–730. Bibcode:2008RvMP...80..633M. doi:10.1103/RevModPhys.80.633.Unknown parameter
|coauthors=ignored (|author=suggested) (help)Direct link to value. - ↑Johnston, Lesley (2008). Salters Advanced Chemistry: Revise Chemistry For Salters AS (Second ed.). Heinemann. p. 2. ISBN978-0-435-63154-3.
- ↑'Avogadro constant'. National Institute of Standards and Technology. Retrieved 2013-11-07.CS1 maint: discouraged parameter (link)
- ↑Andreas, Birk; et al. (2011). 'Determination of the Avogadro Constant by counting the atoms in a 28Si Crystal'. Physical Review Letters. 106 (3). arXiv:1010.2317. Bibcode:2011PhRvL.106c0801A. doi:10.1103/PhysRevLett.106.030801.
Learning Objective
- Define and memorize Avogadro’s number
Key Points
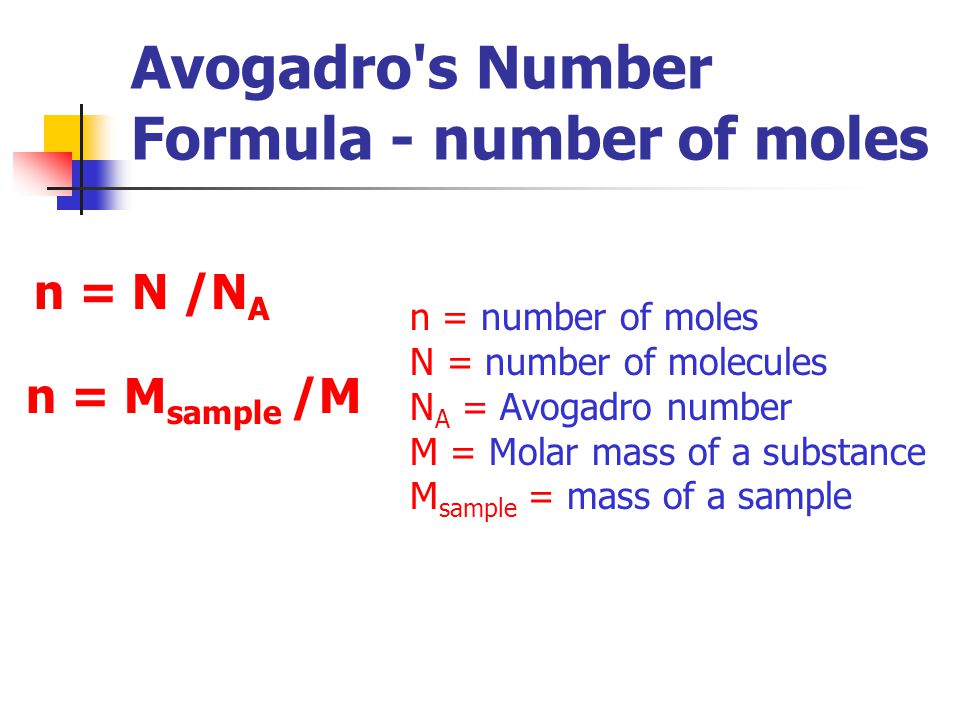
- The mole allows scientists to calculate the number of elementary entities (usually atoms or molecules) in a certain mass of a given substance.
- Avogadro’s number is an absolute number: there are 6.022×1023 elementary entities in 1 mole. This can also be written as 6.022×1023 mol-1.
- The mass of one mole of a substance is equal to that substance’s molecular weight. For example, the mean molecular weight of water is 18.015 atomic mass units (amu), so one mole of water weight 18.015 grams.
Term
- moleThe amount of substance of a system that contains as many elementary entities as there are atoms in 12 g of carbon-12.
The chemical changes observed in any reaction involve the rearrangement of billions of atoms. It is impractical to try to count or visualize all these atoms, but scientists need some way to refer to the entire quantity. They also need a way to compare these numbers and relate them to the weights of the substances, which they can measure and observe. The solution is the concept of the mole, which is very important in quantitative chemistry.
Avogadro’s Number
Amadeo Avogadro first proposed that the volume of a gas at a given pressure and temperature is proportional to the number of atoms or molecules, regardless of the type of gas. Although he did not determine the exact proportion, he is credited for the idea.
Avogadro’s number is a proportion that relates molar mass on an atomic scale to physical mass on a human scale. Avogadro’s number is defined as the number of elementary particles (molecules, atoms, compounds, etc.) per mole of a substance. It is equal to 6.022×1023 mol-1 and is expressed as the symbol NA.
Avogadro’s number is a similar concept to that of a dozen or a gross. A dozen molecules is 12 molecules. A gross of molecules is 144 molecules. Avogadro’s number is 6.022×1023 molecules. With Avogadro’s number, scientists can discuss and compare very large numbers, which is useful because substances in everyday quantities contain very large numbers of atoms and molecules.
The Mole
The mole (abbreviated mol) is the SI measure of quantity of a “chemical entity,” such as atoms, electrons, or protons. It is defined as the amount of a substance that contains as many particles as there are atoms in 12 grams of pure carbon-12. So, 1 mol contains 6.022×1023 elementary entities of the substance.
Chemical Computations with Avogadro’s Number and the Mole
Avogadro’s number is fundamental to understanding both the makeup of molecules and their interactions and combinations. For example, since one atom of oxygen will combine with two atoms of hydrogen to create one molecule of water (H2O), one mole of oxygen (6.022×1023 of O atoms) will combine with two moles of hydrogen (2 × 6.022×1023 of H atoms) to make one mole of H2O.
Another property of Avogadro’s number is that the mass of one mole of a substance is equal to that substance’s molecular weight. For example, the mean molecular weight of water is 18.015 atomic mass units (amu), so one mole of water weight 18.015 grams. This property simplifies many chemical computations.
If you have 1.25 grams of a molecule with molecular weight of 134.1 g/mol, how many moles of that molecule do you have?
[latex]1.25g times frac{ 1 text{ mole}}{134.1g}=0.0093 text{ moles}.[/latex]
Show SourcesAvogadro's Number Named After
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.
Formula Avogadro's Number
http://www.chem1.com/acad/webtext/intro/int-2.html#SEC2
Steve Lower’s Website
CC BY-SA.
http://en.wiktionary.org/wiki/mole
Wiktionary
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Mole_(unit)
Wikipedia
CC BY-SA 3.0.
http://en.wikipedia.org/wiki/Avogadro_constant
Wikipedia
CC BY-SA 3.0.
Avogadro's Number Na
http://en.wikipedia.org/wiki/Avogadro_constant%23mediaviewer/File:Avogadro_Amedeo.jpg
Wikimedia
Public domain.

